2017/05/15
ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes
<Abstract>
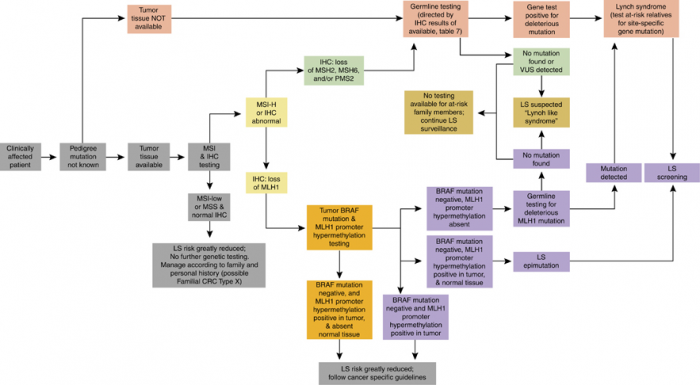
This guideline presents recommendations for the management of patients with hereditary gastrointestinal cancer syndromes. The initial assessment is the collection of a family history of cancers and premalignant gastrointestinal conditions and should provide enough information to develop a preliminary determination of the risk of a familial predisposition to cancer. Age at diagnosis and lineage (maternal and/or paternal) should be documented for all diagnoses, especially in fi rst- and second-degree relatives. When indicated, genetic testing for a germline mutation should be done on the most informative candidate(s) dentifi ed through the family history evaluation and/or tumor analysis to confi rm a diagnosis and allow for predictive testing of at-risk relatives. Genetic testing should be conducted in the context of pre- and post-test genetic counseling to ensure the patient’s informed decision making. Patients who meet clinical criteria for a syndrome as well as those with identifi ed pathogenic germline mutations should receive appropriate surveillance measures in order to minimize their overall risk of developing syndromespecifi c cancers. This guideline specifi cally discusses genetic testing and management of Lynch syndrome, familial adenomatous polyposis FAP), attenuated familial adenomatous polyposis (AFAP), MUTYH -associated polyposis (MAP), Peutz–Jeghers syndrome, juvenile polyposis syndrome, Cowden syndrome, serrated (hyperplastic) polyposis syndrome, hereditary pancreatic cancer, and hereditary gastric cancer.
<Reference>
Am J Gastroenterol 2015; 110:223–262; doi:1 0.1038/ajg.2014.435; published online 3 February 2015